Packaging and storage—
Preserve in tight containers.
Identification—
Constitute Bacampicillin Hydrochloride for Oral Suspension as directed in the labeling. Transfer a portion of the resulting suspension, equivalent to about 140 mg of ampicillin, to a 100-mL volumetric flask, add 70 mL of alcohol, shake by mechanical means for 30 minutes, dilute with alcohol to volume, and mix: the solution so obtained responds to the
Identification test under
Bacampicillin Hydrochloride.
pH  791
791 :
:
between 6.5 and 8.0, in the suspension constituted as directed in the labeling.
Loss on drying  731
731 —
—
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at 60

for 3 hours: it loses not more than 2.0% of its weight.
Assay—
Assay preparation—
Transfer an accurately measured volume of Bacampicillin Hydrochloride for Oral Suspension, constituted as directed in the labeling and free from bubbles, equivalent to about 87.5 mg of ampicillin, to a 250-mL volumetric flask. Add 200 mL of a solvent mixture consisting of alcohol and 0.1 M phosphoric acid (4:1). Shake by mechanical means for 30 minutes, dilute with the same solvent mixture to volume, and mix. Centrifuge a portion of the resulting suspension. Pipet 4.0 mL of the clear solution so obtained into each of two glass-stoppered, 125-mL conical flasks.
Procedure—
Proceed as directed for
Procedure under
Iodometric Assay—Antibiotics  425
425
. Calculate the quantity, in mg, of C
16H
19N
3O
4S in each mL of the constituted Bacampicillin Hydrochloride for Oral Suspension taken by the formula:
(0.0625
F)(
B 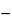 I
I)
/ V,
in which
V is the volume, in mL, of constituted Bacampicillin Hydrochloride for Oral Suspension taken, and the other terms are as defined therein.