Botanic characteristics—
Unground Rauwolfia Serpentina root—
This occurs as segments usually from 5 to 15 cm in length (pieces sometimes shorter) and from 3 to 20 mm in diameter. The pieces are subcylindrical to tapering, rather tortuous or curved, rarely branched, but bearing occasional twisted rootlets, which are larger, more abundant, and more rigid and woody on the thicker parts of the roots. Externally, light brown to grayish yellow to grayish brown, dull, rough or slightly wrinkled longitudinally yet peculiarly smooth to the touch, occasionally showing small circular rootlet scars in the larger pieces, with some exfoliation of the bark in small areas to reveal the paler wood beneath. When scraped, the bark separates readily from the wood. Fracture short, but irregular, the longer pieces readily breaking with a snap, slightly fibrous marginally. The freshly fractured surfaces show a rather thin layer of grayish yellow bark, with the pale yellowish white wood constituting about 80% of the radius. The smoothed transverse surface of larger pieces shows a finely radiate stele with three or more clearly marked growth rings; a small knob-like protuberance is frequently noticeable at the center. The wood is hard and of relatively low density. The odor is indistinct, earthy, reminiscent of stored white potatoes.
Histology—
A transverse section of Rauwolfia Serpentina root shows externally two to eight alternating strata of cork cells, the strata with larger cells alternating with strata made up of markedly smaller cells (distinction from R. canescens). Each stratum composed of smaller cells includes from three to five tangentially arranged cell layers, while each stratum made up of larger cells includes from one to six tangential layers. In a cross-sectional view, the largest central cells of the larger cell group measure 40 to 90 µm radially and up to 75 µm tangentially (although usually smaller), while the cells of the smaller cell groups measure about 5 to 20 µm radially and up to 75 µm tangentially. The walls are thin and suberized. The secondary cortex consists of several rows of tangentially elongated to isodiametric parenchyma cells, most being densely filled with starch grains; others (the short latex cells) occur singly or in short series and contain brown resin masses. The secondary phloem is relatively narrow and is made up of phloem parenchyma (bearing starch grains and less commonly tabular to angular calcium oxalate crystals up to 20 µm in length; also, occasionally, with some brown resin masses in outer cells and phloem rays) interlaid with scattered sieve tissue and traversed by phloem rays two to four cells in width. Sclerenchyma cells (stone cells and fibers) are absent in root (distinction from other species of Rauwolfia). Cambium is indistinct, narrow, dark, and wavering. The secondary xylem represents the large bulk of the root and shows one or more prominent annual rings with a denser core of wood about 500 µm across at the center. The xylem is composed of many wood wedges separated by xylem rays, and on closer examination reveals vessels in interrupted radial rows, much xylem parenchyma, many large-celled xylem rays, few wood fibers, and tracheids, all lignified-walled. The xylem fibers occur in both tangential and radial rows. The xylem rays are 1 to 12, occasionally up to 16, cells in width.
Rauwolfia Serpentina rhizome—Histology—
This is similar to that of root except for the presence of a prominent cortex, pericycle fibers, bicollateral vascular bundles, and a small central pith. The pericycle fibers occur singly or in groups of two to five, have thick, nonlignified walls, tapering, often lobed ends, with subterminal enlargements having thin walls and broad lumina. Vessel elements up to 485 µm are found. The xylem rays are one to four cells in width, with lignified and pitted walls. Internal phloem strands occur embedded in the outer region of the pith. The xylem fibers are somewhat less wavy than those of the root. The pith consists of starch parenchyma cells, among which are scattered short latex cells with yellowish contents stained brown with
iodine TS.
Ground Rauwolfia Serpentina root—
This is brownish to reddish gray in color. Present are very numerous starch grains (mostly simple, two- to three-compound, occasionally four-compound); simple grains spheroid, ovate, muller-shaped, plano- to angular-convex, or irregular; hilum simple, Y-shaped, stellate, or irregularly cleft; unaltered grains 6 to 34 µm (average 20 µm) in diameter, mostly in the lower range (maximum sizes larger than in R. canescens and R. micrantha); altered grains up to about 50 µm in diameter; large unaltered grains show polarization cross clearly; calcium oxalate prisms and cluster crystals scattered, about 10 to 15 µm in size; brown resin masses and yellowish granular secretion masses occur occasionally; isolated cork cells elongated, up to 90 µm in length; phelloderm and phloem parenchyma cells similar in appearance; vessels subcylindrical, up to 360 µm in length and from about 20 µm up to 57 µm in diameter (narrower than in R. canescens) (the wall markings generally consist of simple pits, with bordered pits adjacent to xylem ray cells), the vessel end walls oblique to transverse, generally with openings in the end walls, some vessels showing tyloses; tracheids pitted, with moderately thick, tapering, beaded walls, with relatively broad lumina, polygonal in cross-section; xylem parenchyma cells with moderately thick walls with simple circular pits, cells polygonal in cross-section, bearing considerable starch; phloem and xylem-ray cells with pitted walls bearing much starch, sometimes with brown resin masses, xylem fibers with thick heavily lignified walls showing small transverse and oblique linear pits and pointed simple to bifurcate ends, measuring from 200 to 750 µm in length (shorter than in R. micrantha and R. canescens). No phloem fibers or sclereids are present in root (colorless nonlignified pericycle or primary phloem fibers, single or in small groups, may be present from rhizome or stem tissues).
Chemical identification—
[NOTE—In this procedure, use formamide treated as directed in the specifications for Formamide (see under
Reagents in the section
Reagents,
Indicators,
and Solutions) if it has an ammoniacal odor.
]
Immobile solvent—
Dilute 30 mL of formamide with acetone to 100 mL.
Mobile solvent A—
Mix 90 mL of isooctane, 60 mL of carbon tetrachloride, 4 mL of piperidine, and 2 mL of tertiary butyl alcohol.
Mobile solvent B—
Mix 75 mL of chloroform, 75 mL of isooctane, and 2 mL of tertiary butyl alcohol.
Spray solution—
Dissolve 25 g of trichloroacetic acid in 100 mL of methanol.
Standard solution—
Warm a 1-g portion of
USP Rauwolfia Serpentina RS with 5 mL of alcohol at 55

to 65

for 30 minutes, with occasional mixing. Cool, and filter.
Test preparation—
Reduce 10 g of Rauwolfia Serpentina root to a fine powder. Treat a 1-g portion as in the preparation of the Standard solution.
Procedure A—
Line the sides of a chromatographic chamber suitable for ascending chromatography (see
Chromatography  621
621
) with blotting paper. Transfer
Mobile solvent A to the bottom of the container, and cover the chamber. Immerse a 20- × 20-cm sheet of filter paper (Whatman No. 1 or equivalent) in the
Immobile solvent, and blot between paper toweling. Allow the acetone solvent to evaporate completely. Apply about 1-µL portions of the
Test preparation and of the
Standard solution to a line 2.5 cm from the bottom of the filter paper. Allow to dry. Apply a 2-µL portion of the
Immobile solvent to each spot, allow to dry, and suspend the paper so that it dips into the
Mobile solvent. Cover the chamber, and after about 1 hour, when the
Mobile solvent has risen approximately seven-eighths of the height of the paper, remove the chromatogram, and dry at 90

in a current of air. Spray the paper lightly and evenly with the
Spray solution, and dry at 90

for 10 minutes.
Procedure B—
Use the apparatus described in Procedure A, but containing a glass trough with about 2 mL of ammonium hydroxide to saturate the atmosphere of the tank with NH3. Transfer Mobile solvent B to the bottom of the tank outside the trough. Complete the test as described in Procedure A, omitting the trichloroacetic acid spray. Examine both chromatograms under UV light, and note the fluorescent spots. In both chromatograms the Test preparation yields spots corresponding in position and color to those of the Standard solution.
Assay—
Apparatus—
A medium-sized continuous-extraction apparatus provided with a 250-mL flask and a 35- × 80-mm thimble is convenient, although a smaller apparatus may be used.
Solvents:
alcohol, chloroform, and 1,1,1-trichloroethane. Use 1,1,1-trichloroethane having a boiling range between 73

and 76

.
Standard solution—
Dissolve 20.0 mg of
USP Reserpine RS in 25 mL of hot alcohol, cool, dilute with alcohol to 50.0 mL, and mix. When stored in a tightly-stoppered, light-resistant bottle in the dark, this solution is chromogenically stable for several weeks. Dilute 5.0 mL with alcohol to 100.0 mL, and mix before using.
Procedure—
Extract about 2.5 g of finely powdered Rauwolfia Serpentina, accurately weighed, in a continuous-extraction apparatus for 4 hours. Use about 100 mL of vigorously boiling alcohol as solvent, and a few boiling chips to prevent bumping. Protect the flask and thimble and all solutions of Rauwolfia Serpentina alkaloids from direct or strong light. Wash the extract into a 100-mL volumetric flask with alcohol, cool, dilute with alcohol to volume, and mix. Transfer 20.0 mL to a separator containing 200 mL of 0.5 N sulfuric acid, mix, and extract with three 25-mL portions of 1,1,1-trichloroethane. Lubricate stopcocks only with lubricants insoluble in trichloroethane or chloroform (polytef stopcocks are satisfactory). Drain the lower phase as completely as possible. Wash each of the 1,1,1-trichloroethane extracts in a second separator containing 50 mL of 0.5 N sulfuric acid, and discard the trichloroethane extracts. Extract the weakly basic alkaloids from the first acid solution with 25-, 15-, 15-, 10-, 10-, and 10-mL portions of chloroform. Wash each chloroform extract with the acid in the second separator, then with two 10-mL portions of sodium bicarbonate solution (1 in 50) in two additional separators. Filter the chloroform extracts through chloroform-washed cotton into a 100-mL volumetric flask containing 10 mL of alcohol. Dilute with alcohol to volume, and mix. Transfer duplicate 10.0-mL aliquots to glass-stoppered, 25-mL conical flasks, and mix with 4 mL of alcohol. Evaporate with gentle heating almost to dryness, place in a vacuum desiccator, and evaporate to dryness. Dissolve the residues by agitating with 5.0 mL of alcohol. Transfer duplicate 5.0-mL aliquots of the
Standard solution to flasks. Add 2.0 mL of 0.5 N sulfuric acid to one of the test specimen flasks and to one of the standard flasks (the blanks). Add to the other flasks 1.0 mL of 0.5 N sulfuric acid and 1.0 mL of sodium nitrite solution (3 in 1000). Mix the contents of each flask, and warm in a water bath at 50

to 60

for 20 minutes. Cool, add to each flask 500 µL of sulfamic acid solution (1 in 20), and mix. After stabilization of the solution colors, determine their absorbances in 1-cm cells at 390 nm, relative to a blank consisting of a mixture of alcohol and water (2:1). The quantity, in mg, of reserpine-rescinnamine group alkaloids in the specimen taken is given by the formula:
5(
A 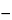 A0
A0) / (
S 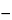 S0
S0),
in which
A and
A0 are the absorbances of the nitrite-treated specimen and specimen blank, respectively, and
S and
S0 are the corresponding absorbances for the solutions from the respective
Standard solution aliquots.