Standard preparation—
Dissolve 25.0 mg of
USP Reserpine RS, accurately weighed, in 0.25 mL of chloroform, mix with about 30 mL of methanol previously warmed to 50

, transfer the mixture to a 250-mL volumetric flask with the aid of warm methanol, cool the solution to room temperature, dilute with methanol to volume, and mix (
Solution 1). Protect the solution from light. Just prior to use in the
Assay, pipet 10 mL of
Solution 1 into a 50-mL volumetric flask, add 36 mL of chloroform, and dilute with methanol to volume (
Standard preparation).
Procedure—
Transfer an accurately measured volume of Oral Solution, equivalent to about 1 mg of reserpine, into a separator or a suitably stoppered, 50-mL centrifuge tube, add 5 mL of citric acid solution (1 in 50) and 10 mL of chloroform, and shake for 2 minutes. Separate and withdraw the chloroform. Wash the citric acid solution with two 10-mL portions of chloroform, adding the washings to the main chloroform solution. To the combined chloroform solutions add 10 mL of sodium bicarbonate solution (1 in 100), shake for 2 minutes, and separate. Withdraw the chloroform, filtering it through a pledget of cotton, into a 50-mL volumetric flask containing 14.0 mL of methanol. Extract the aqueous bicarbonate layer in the extraction vessel with two 2-mL portions of chloroform, passing each portion successively through the filter into the volumetric flask. Add chloroform to volume, and mix (
Assay preparation).
Pipet duplicate 5-mL aliquots of the chloroform-methanol solution and of the Standard preparation into separate, 10-mL volumetric flasks. Add 2.0 mL of a 1 in 10 solution of hydrochloric acid in methanol to each flask. To one flask of each pair of duplicates (representing the Standard preparation and the extracted Assay preparation) add 1.0 mL of a 3 in 1000 solution of sodium nitrite in dilute methanol (1 in 2). To the second flask of each pair (constituting the blanks) add 1 mL of dilute methanol (1 in 2). Mix, and allow to stand for 30 minutes. Add 0.5 mL of ammonium sulfamate solution (1 in 20) to each flask, add methanol to volume, mix, and allow to stand for 10 minutes. Determine the absorbance of each solution in a 1-cm cell at the wavelength of maximum absorbance at about 390 nm, with a suitable spectrophotometer, using a mixture of methanol, chloroform, and water (5.4:3.6:1) as the blank. Calculate the quantity, in mg, of reserpine (C33H40N2O9) in each mL of the Oral Solution taken by the formula:
(
A 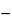 A0
A0)
U / V(
A 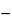 A0
A0)
S,
in which V is the volume, in mL, of Oral Solution taken; and the parenthetic expressions are the differences in absorbances of the nitrite-treated and blank solutions, respectively, from the Assay preparation (U) and the Standard preparation (S).