Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
Transfer a quantity of finely powdered Tablets, equivalent to about 100 mg of hydralazine hydrochloride, to a glass-stoppered flask. Add 40 mL of dilute hydrochloric acid (1 in 12), shake by mechanical means for 5 minutes, and filter, discarding the first few mL of the filtrate. Transfer 20 mL of the filtrate to a separator. Extract with a 10-mL portion of methylene chloride, and discard the methylene chloride. To the aqueous phase add 2 mL of sodium nitrite solution (14 in 1000) and 10 mL of methylene chloride, and shake by mechanical means for 5 minutes. Allow the layers to separate, and drain the methylene chloride through sodium sulfate that has been pre-washed with methylene chloride, into a 50-mL beaker. Evaporate over gentle heat with the aid of nitrogen to dryness: the IR absorption spectrum of a potassium bromide dispersion of the residue so obtained exhibits maxima only at the same wavelengths as that of a similar preparation of
USP Hydralazine Hydrochloride RS, similarly treated (
presence of hydralazine hydrochloride)
.
B:
Transfer a quantity of finely powdered Tablets, equivalent to about 1 mg of reserpine, to a 50-mL centrifuge tube. Add 20 mL of cyclohexane, shake by mechanical means for 15 minutes, centrifuge, and discard the cyclohexane. Repeat the extraction with two additional 20-mL portions of cyclohexane, shaking by mechanical means for 2 minutes each time. To the residue add 10 mL of chloroform, shake for 2 minutes, and filter through a medium-porosity, sintered-glass funnel into another 50-mL centrifuge tube. Extract the chloroform with 10 mL of 1.0 N hydrochloric acid, discarding the aqueous acid layer. Extract the chloroform with 10 mL of 0.5 N sodium hydroxide. Centrifuge for 5 minutes, and withdraw the chloroform with a syringe, passing the chloroform through cotton into a 50-mL volumetric flask containing 40 mL of methanol. Dilute with chloroform to volume, and mix: the UV absorption spectrum of the solution so obtained exhibits maxima and minima at the same wavelengths as that of a similar solution of
USP Reserpine RS, concomitantly measured (
presence of reserpine)
.
C:
The UV absorption spectrum of the solution from the Assay preparation, obtained as directed for Procedure in the Assay for hydrochlorothiazide, exhibits maxima and minima at the same wavelengths as that of the solution from the Standard preparation, prepared as directed in the Assay for hydrochlorothiazide, similarly measured (presence of hydrochlorothiazide).
Uniformity of dosage units  905
905 :
:
meet the requirements for
Content Uniformity with respect to reserpine, to hydralazine hydrochloride, and to hydrochlorothiazide.
Procedure for content uniformity—
Apparatus—
Use an automatic analyzer consisting of (1) a solid sampler with 100-mL dissolution capability; (2) a 20-channel peristaltic pump; (3) a continuous filtering device; (4) a colorimeter equipped with a 2-mm flow cell and analysis capability at 530 nm; (5) a UV spectrophotometer equipped with a 10-mm flow cell and analysis capability at 271 nm; (6) a spectrofluorometer equipped with a 2-mm flow cell and analysis capability of 365 nm activation energy and 495 nm fluorescence; (7) recording devices for each of the three aforementioned detectors; and (8) a manifold consisting of components illustrated in the pertinent diagram in the chapter
Automated Methods of Analysis  16
16
. Prepare the ion-exchange column listed in the manifold as follows. Wash sulfonic acid cation-exchange resin (40- to 60-mesh) with isopropyl alcohol until the alcohol shows no appreciable UV absorption at 271 nm, then add sufficient 1 N hydrochloric acid to cover the resin. Drain off the acid, and wash the resin with an equivalent volume of 1 N sodium hydroxide and then again with 1 N hydrochloric acid. Finally wash the resin with water until the effluent is neutral to litmus. Draw the resin into a 10-mm length of “solvaflex” tubing (1 mm ID) by vacuum, and plug each end with glass wool.
Saturated vanadium pentoxide
(V2O5)—Stir for about 6 hours or shake by mechanical means for 2 hours about 100 mg of vanadium pentoxide powder with 100 mL of phosphoric acid. Filter through a medium-porosity, sintered-glass funnel. This solution is stable for 1 month.
0.3% Hydrogen peroxide
(H2O2)—Dilute 1 mL of 30 percent hydrogen peroxide with water to 100 mL. Prepare fresh daily.
Blue tetrazolium reagent
(B.T.)—Dissolve 760 mg of blue tetrazolium in 3.8 liters of a mixture of dehydrated alcohol and methanol (19:1).
Tetramethylammonium hydroxide
(T.M.A.H.)—Dilute 38 mL of tetramethylammonium hydroxide solution (1 in 10) with dehydrated alcohol and methanol (19:1) to 3.8 liters.
Solvent, wash, and diluent—
Prepare by mixing equal volumes of methanol and water. Add 1 mL of phosphoric acid to each 3.8 liters.
Standard preparation—
Accurately weigh about 42.0 mg of
USP Reserpine RS into a 200-mL volumetric flask, dissolve in methanol, and dilute with methanol to volume. Pipet 10 mL of this solution into a 100-mL volumetric flask containing about 315.0 mg of
USP Hydrochlorothiazide RS and about 525.0 mg of
USP Hydralazine Hydrochloride RS, accurately weighed. Dissolve in
Solvent, and dilute with
Solvent to volume. (A 5-mL aliquot represents 1 standard Tablet.)
Procedure—
With the sampler in the standby position, pump all reagents through the system until a stable baseline is achieved. Activate the sampler, and allow one cycle to pass without introducing the Tablets or the
Standard preparation, then pipet a 5-mL aliquot of the
Standard preparation into the hopper at the solvent addition portion for the next two cycles and for every sixth cycle thereafter. Disregard the first value for the
Standard preparation. Add the Tablets to the sampler at the rate of 20 per hour to follow the second 5-mL aliquot of the
Standard preparation. Record the absorbance and fluorescence values, and calculate each peak value by the difference between peak height and baseline. Calculate the quantity, in mg per Tablet, taken by the formula:
(0.005 / 1.05)C(AU / AS),
in which
C is the concentration, in µg per mL, of the appropriate Reference Standard in the
Standard preparation, AU is the absorbance of the Tablet, and
AS is the averaged absorbance of the
Standard preparations.
Diazotizable substances—
Standard solution—
Weigh accurately 25 mg of
USP Benzothiadiazine Related Compound A RS, dissolve in 5 mL of methanol contained in a 50-mL volumetric flask, dilute with water to volume, and mix. Pipet 4 mL of this solution into a 100-mL volumetric flask, dilute with water to volume, and mix.
Test solution—
Transfer a portion of the powdered Tablets prepared for the Assay for hydrochlorothiazide, accurately weighed and equivalent to about 100 mg of hydrochlorothiazide, to a 100-mL volumetric flask, and add a mixture of 20 mL of methanol and 20 mL of water. Shake continuously for 5 to 10 minutes, dilute with water to volume, mix, and filter. Use the filtrate as the Test solution.
Procedure—
Pipet 5 mL each of the
Standard solution and the
Test solution into separate, 50-mL volumetric flasks. Pipet 5 mL of water into a third 50-mL volumetric flask to provide the blank. To each flask add 1 mL of freshly prepared sodium nitrite solution (1 in 100) and 5 mL of dilute hydrochloric acid (1 in 12), and allow to stand for 5 minutes. Add 2 mL of ammonium sulfamate solution (1 in 50), allow to stand for 5 minutes with frequent swirling, then add 2 mL of freshly prepared disodium chromotropate solution (1 in 100) and 10 mL of
sodium acetate TS. Dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions from the
Standard solution and the
Test solution in 1-cm cells at the wavelength of maximum absorbance at about 500 nm, with a suitable spectrophotometer, against the blank. The absorbance of the solution from the
Test solution does not exceed that of the solution from the
Standard solution, corresponding to not more than 2.0% of diazotizable substances.
Assay for reserpine—
Standard preparation—
Dissolve about 25 mg of
USP Reserpine RS, accurately weighed, in chloroform in a 50-mL volumetric flask, and dilute with chloroform to volume (
Solution 1). Pipet 10 mL of
Solution 1 into a 50-mL volumetric flask, and dilute with chloroform to volume. Protect the solution from light.
Assay preparation—
Weigh and finely powder not less than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 1 mg of reserpine, to a separator or a stoppered, 50-mL centrifuge tube containing 10 mL of citric acid solution (1 in 50). Shake vigorously until the powder is completely suspended. Add 10 mL of chloroform, and proceed as directed for Procedure, beginning with “shake thoroughly for 2 minutes.”
Procedure—
[NOTE—Conduct the entire procedure quickly, without exposure to direct sunlight. Perform the extractions in a suitable separator or in a suitably stoppered, 50-mL centrifuge tube, and separate by centrifuging, withdrawing the portion to be retained into a hypodermic syringe fitted with a square-tipped, 14-gauge, 15-cm needle.
] Pipet 10 mL of the
Standard preparation into the extraction vessel, add 10 mL of citric acid solution (1 in 50), and shake thoroughly for 2 minutes. Separate and withdraw the chloroform. Wash the citric acid solution with two 10-mL portions of chloroform, adding the washings to the main chloroform solution. To the combined chloroform solutions add 10 mL of citric acid solution (1 in 50), shake for 2 minutes, and separate and withdraw the chloroform. To the combined chloroform solutions add 10 mL of sodium bicarbonate solution (1 in 100), shake for 2 minutes, and separate. Withdraw the chloroform, filtering it through a pledget of cotton, into a 50-mL volumetric flask containing 14.0 mL of methanol. Extract the aqueous bicarbonate layer in the extraction vessel with two 2-mL portions of chloroform, passing each portion successively through the filter into the volumetric flask. Add chloroform to volume, and mix. Pipet duplicate 5-mL aliquots of the chloroform-methanol solutions into separate, 10-mL volumetric flasks. Add 2.0 mL of a 1 in 10 solution of hydrochloric acid in methanol to each flask. To one flask of each pair of duplicates (representing the extracted
Standard preparation and the extracted
Assay preparation) add 1.0 mL of a 3 in 1000 solution of sodium nitrite in dilute methanol (1 in 2). To the second flask of each pair (constituting the blanks) add 1 mL of dilute methanol (1 in 2). Mix, and allow to stand for 30 minutes. Add 0.5 mL of ammonium sulfamate solution (1 in 20) to each flask, add methanol to volume, mix, and allow to stand for 10 minutes. Determine the absorbance of each solution in a 1-cm cell at the wavelength of maximum absorbance at about 390 nm, with a suitable spectrophotometer, relative to the absorbance of a mixture of methanol, chloroform, and water (5.4:3.6:1).
Calculate the quantity, in mg, of C33H40N2O9 in the portion of Tablets taken by the formula:
0.01
C(
A 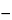 A0
A0)
U / (
A 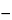 A0
A0)
S,
in which
C is the concentration, in µg per mL, of
USP Reserpine RS in the
Standard preparation, and the parenthetic expressions are the differences in absorbances of the nitrite-treated and blank solutions, respectively, from the
Assay preparation (
U) and the
Standard preparation (
S).
Assay for hydralazine hydrochloride—
Sodium acetate solution—
Dissolve 27.2 g of sodium acetate trihydrate in 50 mL of water, bring to room temperature, and dilute with water to 100 mL.
Ferric ammonium sulfate solution—
Dissolve 1.8 g of ferric ammonium sulfate in 4 mL of dilute hydrochloric acid (1 in 12), and dilute with water to 100 mL. Filter, and use the clear filtrate. Prepare fresh.
1,10-Phenanthroline solution—
Shake 300 mg of 1,10-phenanthroline with 100 mL of water for 1 hour. Filter, and use the clear filtrate. Prepare fresh.
Standard preparation—
Dissolve about 50 mg of
USP Hydralazine Hydrochloride RS, accurately weighed, in water in a 100-mL volumetric flask, and dilute with water to volume. Pipet 10 mL of this solution into a 100-mL volumetric flask, dilute with water to volume, and mix.
Assay preparation—
Weigh and finely powder not less than 20 Tablets. Transfer to a 200-mL volumetric flask an accurately weighed portion of the powder, equivalent to about 100 mg of hydralazine hydrochloride, add 100 mL of water, and shake by mechanical means for 30 minutes. Dilute with water to volume, mix, and filter through paper, discarding the first 15 mL of the filtrate. Pipet 10 mL of the clear filtrate into a 100-mL volumetric flask, and dilute with water to volume.
Procedure—
Pipet 10 mL each of the
Assay preparation, the
Standard preparation, and water to provide the blank, into separate, 200-mL volumetric flasks. To each flask add 5 mL of acetic acid solution (12 in 100), 5 mL of
Sodium acetate solution, 2 mL of
1,10-Phenanthroline solution, and 1 mL of
Ferric ammonium sulfate solution, mix, and allow to stand in the dark for 30 minutes. Dilute with water to volume, and mix. Concomitantly determine the absorbances of both solutions in 1-cm cells at the wavelength of maximum absorbance at about 510 nm, with a suitable spectrophotometer, against the blank. Calculate the quantity, in mg, of C
8H
8N
4·HCl in the portion of Tablets taken by the formula:
2C(AU / AS),
in which
C is the concentration, in µg per mL, of
USP Hydralazine Hydrochloride RS in the
Standard preparation, and
AU and
AS are the absorbances of the solutions from the
Assay preparation and the
Standard preparation, respectively.
Assay for hydrochlorothiazide—
Methanolic sodium hydroxide solution—
Dissolve 40 g of sodium hydroxide in 125 mL of water, cool, and dilute with methanol to 500 mL. Filter before use.
Column preparation—
Weigh about 10 g of sulfonic acid cation exchange resin into a 250-mL beaker. Add 75 mL of methanol, and stir the mixture with a magnetic stirrer for 30 minutes. Place a glass wool plug at the lower end of a glass chromatographic column (15 mm × 45-cm long), equipped with a stopcock to regulate the eluant flow. Transfer and pack the slurry in portions into the prepared column to a height of 10 cm. If air is trapped, remove by tapping, stirring, or back-flushing the column. Place a glass wool plug on top of the resin bed after packing. Wash the resin with consecutive 100-mL portions of a 1 in 5 solution of hydrochloric acid in methanol, and of methanol, then of Methanolic sodium hydroxide solution and of methanol, respectively, using a flow rate of approximately 4 mL per minute. Wash the sodium form of the resin with 150 mL of a 1 in 5 solution of hydrochloric acid in methanol, followed by not less than 100 mL of methanol. The UV spectrum of the last few mL of the methanol washing conforms to that of the methanol.
Standard preparation—
Mix about 40 mg of
USP Hydrochlorothiazide RS, accurately weighed, with 4 mL of water in a 200-mL volumetric flask. Add 150 mL of methanol, warm gently on a steam bath for a few minutes, and shake by mechanical means for 20 minutes. Dilute with methanol to volume, and mix.
Assay preparation—
Weigh and finely powder not less than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 20 mg of hydrochlorothiazide, to a 100-mL volumetric flask. Add 2 mL of water, mix, and add 75 mL of methanol. Warm gently on a steam bath for a few minutes, and shake by mechanical means for 20 minutes. Dilute with methanol to volume, and mix. Centrifuge a portion of the mixture, and use the clear solution as the Assay preparation.
Procedure—
Keeping the column stopcocks closed, pipet 4 mL of the clear
Assay preparation on the top of the resin bed of 1 column, and pipet 4 mL of the
Standard preparation on the top of the second column. Place a 100-mL volumetric flask beneath each of the columns, and collect the eluate. Adjust the flow rate to approximately 2 mL per minute, and allow the solution to flow until it just disappears into the upper glass wool plug. Close the stopcock, carefully add 25 mL of methanol, and further elute each column with the same flow rate as before. Repeat with two additional 25-mL portions of methanol. Dilute the contents of each flask with methanol to volume. Concomitantly determine the absorbances of both solutions at the wavelength of maximum absorbance at about 271 nm, with a suitable spectrophotometer, using methanol as the blank. Calculate the quantity, in mg, of C
7H
8ClN
3O
4S
2 in the portion of Tablets taken by the formula:
0.1C(AU / AS),
in which
C is the concentration, in µg per mL, of
USP Hydrochlorothiazide RS in the
Standard preparation, and
AU and
AS are the absorbances of the solution from the
Assay preparation and the
Standard preparation, respectively.