Identification—
A:
Transfer a volume of Injection, equivalent to about 100 mg of secobarbital sodium, to a separator containing 15 mL of water. Render the mixture distinctly acid to litmus with hydrochloric acid, extract the liberated secobarbital with 25 mL of ether, collect the ether extract in a separator, and wash with 10 mL of water. Discard the water solution. Filter the ether extract into a beaker, and evaporate on a steam bath with the aid of a current of air just to dryness. Dissolve the residue in 3 mL of alcohol, and evaporate to dryness. Repeat the dissolution and evaporation with 3 mL of alcohol, and dry the residue at 100

for 2 hours: the IR absorption spectrum of a solution prepared by dissolving the residue of secobarbital so obtained in chloroform to a concentration of about 50 mg per mL, 0.1-mm sodium chloride cells being used and chloroform being used as the blank, exhibits maxima only at the same wavelengths as that of a similar preparation of
USP Secobarbital RS.
B:
It responds to the flame test for
Sodium  191
191
.
Assay—
Buffer solution—
Dissolve 6.19 g of boric acid and 14.91 g of potassium chloride in water, dilute with water to 200 mL, and mix. After 24 hours, filter if necessary to obtain a clear solution.
Standard preparation 1—
Dissolve a suitable quantity of
USP Secobarbital RS, accurately weighed, in 0.5 N sodium hydroxide to obtain a solution having a known concentration of about 23 µg per mL.
Standard preparation 2—
Mix 5.0 mL of Standard preparation 1 with 5.0 mL of Buffer solution.
Assay preparation 1—
Transfer an accurately measured volume of Injection, equivalent to about 50 mg of secobarbital sodium, to a 100-mL volumetric flask, dilute with 0.5 N sodium hydroxide to volume, and mix. Pipet 5 mL of this solution into a 100-mL volumetric flask, add 0.5 N sodium hydroxide to volume, and mix.
Assay preparation 2—
Mix 5.0 mL of Assay preparation 1 with 5.0 mL of Buffer solution.
Procedure—
Concomitantly determine the absorbances of
Assay preparation 1 and
Standard preparation 1 in 1-cm cells at 260 nm, with a suitable spectrophotometer, using 0.5 N sodium hydroxide as the blank. Similarly determine the absorbances of
Assay preparation 2 and
Standard preparation 2, using as the blank a mixture of equal volumes of 0.5 N sodium hydroxide and
Buffer solution. Calculate the quantity, in mg, of C
12H
17N
2NaO
3 in the volume of Injection taken by the formula:
1.092(2
C)(
AU 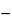
2
aU)
/ (
AS 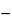
2
aS),
in which 1.092 is the ratio of the molecular weight of sodium secobarbital to that of secobarbital,
C is the concentration, in µg per mL, of
USP Secobarbital RS in
Standard preparation 1,
AU and
AS are the absorbances of
Assay preparation 1 and
Standard preparation 1, respectively, and
aU and
aS are the absorbances of
Assay preparation 2 and
Standard preparation 2, respectively.