Packaging and storage—
Preserve and dispense in the containers in which it was placed by the manufacturer. Keep liquid Vaccine during storage and in shipment at a temperature below 0

. Keep dried Vaccine at a temperature between 2

and 8

.
Expiration date—
The expiration date for liquid Vaccine is not later than 3 months after date of issue from manufacturer's cold storage (
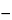
10

, 9 months as glycerinated or equivalent preparation). The expiration date for dried Vaccine is not later than 18 months after date of issue from manufacturer's cold storage (5

, 6 months).
Labeling—
Label it to state that it contains not more than 200 microorganisms per mL in the case of Vaccine intended for multiple-puncture administration, or that it contains not more than 1 microorganism per 100 doses in the case of Vaccine intended for jet injection, unless it meets the requirements for sterility. In the case of Vaccine intended for jet injection, so state on the label. In the case of dried Vaccine, label it to state that after constitution it is to be well shaken before use. Label it also to state that it was prepared in the bovine calf.