Sodium content—
Sodium solution—
Dissolve in water an accurately weighed quantity of sodium chloride to make a solution containing 5.00 mg of sodium per mL.
Standard graph—
Into four 1-liter flasks pipet, respectively, 0, 1, 2, and 3 mL of Sodium solution. To each flask add 0.1 mL of nitric acid, 0.1 mL of sulfuric acid, and 10 mL of low-sodium, low-potassium, nonionic surfactant solution (1 in 50), dilute with water to volume, and mix. Adjust the scale of a suitable flame spectrophotometer to a reading of 100 at a wavelength of 588 nm with the solution containing 15 mg of sodium per L. Determine the instrument readings on the other three solutions, and plot the observed readings, on ruled coordinate paper, as the ordinate, and the concentration of sodium, in mg per liter, as the abscissa. The line intersects the ordinate at, or below, a scale reading of 25 (“blank reading”).
Procedure—
Ash the equivalent of 1 g of Sodium Polystyrene Sulfonate, accurately weighed, with a slight excess of sulfuric acid. Add 1 mL of nitric acid and a few mL of water to the residue. Warm to dissolve, and transfer with water to a 1-liter volumetric flask, dilute with water to volume, and mix. Pipet 10 mL of this solution into a 100-mL volumetric flask, add 1 mL of low-sodium, low-potassium, non-ionic surfactant solution (1 in 50), dilute with water to volume, and mix. Determine the instrument reading concomitantly with the readings obtained for plotting the
Standard graph, and determine the sodium concentration, in mg per liter, by interpolation from the
Standard graph. Calculate the percentage of sodium taken by the formula:
A / W,
in which
A is the weight, in mg, of sodium found per L and
W is the weight, in g, of Sodium Polystyrene Sulfonate taken. The sodium content is not less than 9.4% and not more than 11.5%, calculated on the anhydrous basis.
Potassium exchange capacity—
Potassium solution—
Dissolve an accurately weighed quantity of potassium chloride in water to make a solution containing 5.00 mg of potassium per mL.
Sodium solution—
Dissolve an accurately weighed quantity of sodium chloride in water to make a solution containing 4.00 mg of sodium per mL.
Standard graph—
Identify five 1-liter volumetric flasks by the numbers 1, 2, 3, 4, and 5. In that order pipet into the flasks 4, 3, 2, 1, and 0 mL, respectively, of Sodium solution, and in the same order 0, 1, 2, 3, and 4 mL, respectively, of Potassium solution. To each flask add 10 mL of low-sodium, low-potassium, nonionic surfactant solution (1 in 50), dilute with water to volume, and mix. Adjust the scale of a suitable flame spectrophotometer to 100 with solution from flask 5 at 766 nm. Determine the instrument readings with solutions from flasks 4, 3, 2, and 1. On ruled coordinate paper, plot the observed instrument readings as the ordinate, and the concentrations, in mg per liter, of potassium as the abscissa.
Procedure—
Pipet 100 mL of
Potassium solution into a glass-stoppered flask containing about 1.6 g of Sodium Polystyrene Sulfonate, accurately weighed, shake by mechanical means for 15 minutes, filter, and discard the first 20 mL of the filtrate. Pipet 5 mL of the filtrate into a 1-liter volumetric flask, add 10 mL of low-sodium, low-potassium, nonionic surfactant solution (1 in 50), dilute with water to volume, and mix. Observe the flame spectrophotometer readings of the exchanged solution concomitantly with those obtained for plotting the
Standard graph, and determine the potassium concentration, in mg per liter, by interpolation from the
Standard graph. Calculate the quantity, in mg per g, of potassium adsorbed on the resin taken by the formula:
(
X 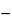
20
Y) /
W,
in which
X is the weight, in mg, of potassium in 100 mL of
Potassium solution before exchange;
Y is the weight, in mg, of potassium per L as interpolated from the
Standard graph; and
W is the weight, in g, of Sodium Polystyrene Sulfonate taken, expressed on the anhydrous basis.