Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
Boil an amount of powdered Tablets, equivalent to about 2 mg of stanozolol, with 5 mL of benzene, filter, and evaporate on a steam bath to dryness. Add 3 mL of p-dimethylaminobenzaldehyde TS to the residue: a yellow color develops, which exhibits a green fluorescence under long-wavelength UV light.
Dissolution  711
711 —
—
Medium:
0.1 N hydrochloric acid; 500 mL.
Apparatus 2:
50 rpm.
Time:
45 minutes.
Determine the amount of C21H32N2O dissolved by employing the following method.
Bromocresol purple solution—
Mix 1.0 g of bromocresol purple with 1000 mL of dilute glacial acetic acid (1 in 50), and filter if necessary to obtain a clear solution.
Standard preparations—
[NOTE—Prepare
Standard preparations on the day of use.
] Transfer about 50 mg of
USP Stanozolol RS, accurately weighed, to a 50-mL volumetric flask, add 15.0 mL of methanol, and mix to dissolve. Add 5.0 mL of 1.0 N hydrochloric acid, dilute with water to volume, and mix. Transfer 5.0 mL of the resulting solution to a 200-mL volumetric flask, dilute with
Dissolution Medium to volume, and mix. Separately pipet 2-mL, 4-mL, and 6-mL portions of the solution into three 60-mL separators, add accurately measured volumes of
Dissolution Medium to adjust the volumes in each to 25.0 mL, and pipet 25 mL of
Dissolution Medium into a fourth 60-mL separator.
Procedure—
Pipet 25 mL of a filtered portion of the solution under test into a 60-mL separator. To this separator and to each of the four separators containing Standard preparations add 1.0 mL of Bromocresol purple solution and 10.0 mL of chloroform. Insert the stopper in each, shake gently for 1 minute, allow the phases to separate, and swirl if necessary to break up emulsions. Transfer the lower chloroform layers to separate 50-mL centrifuge tubes, insert the glass stoppers, and centrifuge for 5 minutes to clarify the solutions. Concomitantly determine the absorbances of the solutions obtained from the solution under test and from the Standard preparation in 1-cm cells, at the wavelength of maximum absorbance at about 420 nm, with a suitable spectrophotometer, using chloroform as the blank. Construct a standard plot of absorbances versus the concentrations of the solutions from the Standard preparations. From the plot so obtained, determine the amount of C21H32N2O dissolved in the solution from the solution under test.
Tolerances—
Not less than 75% (Q) of the labeled amount of C21H32N2O is dissolved in 45 minutes.
Uniformity of dosage units  905
905 —
[NOTE—
—
[NOTE—Maintain the acid concentration at a uniform level in the solutions being compared spectrophotometrically; the same acidic alcohol solution is to be used throughout this procedure. Also, take precautions throughout this procedure to minimize evaporation.
] Transfer 1 Tablet to a 25-mL volumetric flask, add 0.5 mL of water, and shake to disintegrate. Add about 20 mL of alcohol, heat on a steam bath, with occasional swirling, for 10 to 15 minutes, then cool, dilute with alcohol to volume, and mix. Filter through medium-porosity filter paper, taking precautions to minimize evaporation, discard the first 5 mL of the filtrate, and proceed as directed for
Assay preparations in the
Assay, beginning with “Transfer 5.0 mL of the filtrate.”
Assay—
[NOTE—Maintain the acid concentration at a uniform level in the solutions being compared spectrophotometrically; the same acidic alcohol solution is to be used throughout this procedure.
]
Standard preparations—
Dissolve a suitable quantity of
USP Stanozolol RS, accurately weighed, in alcohol, and dilute quantitatively and stepwise with alcohol, if necessary, to obtain a stock solution having a known concentration of about 80 µg per mL. Transfer 5.0 mL of this stock solution to a 10-mL volumetric flask, dilute with alcohol to volume, and mix to prepare the
Neutral standard preparation. Transfer another 5.0-mL portion of the stock solution to a second 10-mL volumetric flask, dilute with acidic alcohol (1.5 mL of hydrochloric acid in 100 mL of alcohol) to volume, and mix to prepare the
Acidic standard preparation. The concentration of
USP Stanozolol RS in the
Standard preparations is about 40 µg per mL.
Assay preparations—
Weigh and finely powder not less than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 4 mg of stanozolol, to a 50-mL volumetric flask, add about 25 mL of alcohol, and heat on a steam bath, with frequent swirling, for 15 minutes. Cool, dilute with alcohol to volume, mix, filter through medium-porosity filter paper, taking precautions to minimize evaporation, and discard the first 10 mL of the filtrate. Transfer 5.0 mL of the filtrate to a 10-mL volumetric flask, dilute with alcohol to volume, and mix to prepare the Neutral assay preparation. Transfer another 5.0-mL portion of the filtrate to a second 10-mL volumetric flask, dilute with acidic alcohol (1.5 mL of hydrochloric acid in 100 mL of alcohol) to volume, and mix to prepare the Acidic assay preparation.
Procedure—
Concomitantly determine the absorbances of the acidic alcohol solution, the
Acidic standard preparation, and the
Acidic assay preparation in 1-cm cells at the wavelength of maximum absorbance at about 235 nm, with a suitable spectrophotometer, using alcohol, the
Neutral standard preparation, and the
Neutral assay preparation, respectively, as the blanks. Calculate the quantity, in mg, of C
21H
32N
2O in the portion of Tablets taken by the formula:
0.1
C(
AU 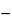 AO
AO)/(
AS 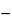 AO
AO),
in which
C is the concentration, in µg per mL, of
USP Stanozolol RS in the
Standard preparations; and
AU,
AS, and
AO are the absorbances of the
Acidic assay preparation, the
Acidic standard preparation, and the acidic alcohol solution, respectively.