Packaging and storage—
Preserve in well-closed containers, protected from light. Store at 25

, excursions permitted between 15

and 30

.
Change to read:
 Dissolution
Dissolution  711
711 —
—
Test 1— USP29
USP29
Medium:
0.3 M phosphate buffer, pH 6.0 (prepared by dissolving 816.5 g of monobasic potassium phosphate and 48 g of sodium hydroxide in about 4 L of water, mixing, and diluting with water to 20 L. Adjust with either concentrated phosphoric acid or 1 N sodium hydroxide to a pH of 6.0 ± 0.05); 900 mL.
Apparatus 2:
75 rpm.
Times:
30, 45, 60, and 120 minutes.
Procedure—
Determine the percentages of the labeled amount of clarithromycin (C38H69NO13) dissolved using the following method.
Standard solutions—
Prepare five solutions of
USP Clarithromycin RS dissolved in acetonitrile and diluted in
Medium, with known concentrations over the range of about 60 to 600 µg per mL.
Test solution—
Use portions of the solution under test passed through a 35-µm polyethylene filter.
Chromatographic system—
Proceed as directed in the Assay.
Procedure—
Separately inject equal volumes (about 50 µL) of the five Standard solutions and the Test solution into the chromatograph, and measure the responses for the major peaks. Perform a linear regression analysis to generate a standard curve using the peak area of each Standard solution versus its concentration. Determine the amount of clarithromycin (C38H69NO13) dissolved at each specified time interval, using the peak area of each Test solution and the linear regression statistics for the Standard solutions.
Tolerances—
The percentages of the labeled amounts of clarithromycin (C
38H
69NO
13) dissolved at the times specified conform to the following
Acceptance Table.
Acceptance Table
| Level |
Time
(minutes) |
 Amount dissolved (individual limits) Amount dissolved (individual limits) |
Amount dissolved (average
limits) |
| L1 |
30 |
not more than 65% |
— |
|
45 |
between 55% and 85% |
— |
|
60 |
not less than 75% |
— |
|
120 |
not less than 85% |
— |
| L2 |
30 |
not more than 75% |
not more than 65% |
|
45 |
between 45% and 95% |
between 55% and 85% |
|
60 |
not less than 65% |
not less than 75% |
|
120 |
not less than 75% |
not less than 85% |
| L3 |
30 |
not more than 2 tablets release more than 75%,
and no individual tablet releases more than 85% |
not more than 65% |
|
45 |
not more than 2 tablets are outside the range
of 45% to 95%, and no individual tablet is
outside the range of 35% to 105% |
between 55% and 85% |
|
60 |
not more than 2 tablets release less than 65%,
and no individual tablet releases less than 55% |
not less than 75% |
|
120 |
not more than 2 tablets release less than 75%,
and no individual tablet releases less than 65% |
not less than 85% |
 Test 2—
Test 2—
Medium:
0.05 M phosphate buffer, pH 6.8 containing 0.5% of sodium lauryl sulfate; 900 mL, degassed by sonication and vacuum.
Apparatus 1:
100 rpm.
Times:
2, 12, and 24 hours.
Procedure—
Determine the percentages of the labeled amount of clarithromycin (C38H69NO13) dissolved using the following method.
0.067 M Phosphate buffer, pH 2.5—
Dissolve 9.2 g of monobasic sodium phosphate monohydrate in about 800 mL of water. Adjust with phosphoric acid to a pH of 2.5. Dilute with water to 1000 mL.
Mobile phase—
Prepare a filtered and degassed mixture of methanol and
0.067 M Phosphate buffer, pH 2.5 (65:35). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard solution—
Transfer about 56 mg of
USP Clarithromycin RS, accurately weighed, to a 100-mL volumetric flask. Add 10 mL of methanol, and sonicate to dissolve. Dilute with
Medium to volume.
Test solution—
Centrifuge the solution under test at 2500 rpm for 10 minutes.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm × 15-cm column that contains 5-µm packing L1. The flow rate is about 1.0 mL per minute. Chromatograph the
Standard solution, and record the peak responses as directed for
Procedure: the tailing factor is not more than 2.0; the column efficiency is not less than 2000; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 5 µL) of the
Standard solution and
Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Determine the amount, in percentage, of clarithromycin dissolved by the formula:
in which
CU is the concentration, in mg per mL, of clarithromycin in the sample at each time point;
rU and
rS are the peak responses obtained from the
Test solution and the
Standard solution, respectively; and
CS is the concentration, in mg per mL, of clarithromycin in the
Standard solution.
Calculate the amount, in percentage, of clarithromycin dissolved with volume correction:
in which
Cn is the concentration, in mg per mL, of clarithromycin in the
Test solution at each time point; 900 is the volume, in mL, of
Medium; VU is the volume, in mL, of sample withdrawn at each time point;
n is the time point (at 2 hours, n = 1), summation of the concentration of the
Test solution from the first to the (
n –1)th time point (only applicable for
n 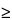
2); 100 is the conversion factor to percentage; and
LC is the Tablet label claim.
Tolerances—
The percentages of the labeled amounts of clarithromycin (C
38H
69NO
13) dissolved at the times specified conform to
Acceptance Table 2.
| Time (hours) |
 Amount dissolved Amount dissolved |
| 2 |
not more than 20% |
| 12 |
between 45% and 70% |
| 24 |
not less than 80% |
 USP29
USP29
Assay—
Mobile phase, Resolution solution, and Chromatographic system—
Proceed as directed in the
Assay under
Clarithromycin Tablets.
Assay preparation—
Finely powder an accurately counted number of Tablets, equivalent to about 2000 mg of clarithromycin. With the aid of methanol quantitatively transfer the powder to a 500-mL volumetric flask, add about 350 mL of methanol, and shake by mechanical means for 30 minutes. Dilute with methanol to volume, and mix. Sonicate for 30 minutes. Cool to room temperature, and allow to stand for at least 16 hours. Mix, and allow any insoluble matter to settle. Transfer 3.0 mL of the supernatant to a 100-mL volumetric flask, dilute with Mobile phase to volume, and mix. Pass a portion of this solution through a filter having a 0.5-µm or finer porosity, and use the filtrate as the Assay preparation.
Procedure—
Proceed as directed for
Procedure in the
Assay under
Clarithromycin Tablets. Calculate the quantity, in mg, of clarithromycin (C
38H
69NO
13) in each Extended-Release Tablet taken by the formula:
(50/3)(C/N)(rU / rS)
in which
N is the number of Tablets taken, and the other terms are as defined therein.