Packaging and storage—
Preserve in adequately shielded single-dose containers in a freezer.
Labeling—
Label it to include the following, in addition to the information specified for
Labeling under
Injections  1
1
: the time and date of calibration; the amount of
153Sm complexed with EDTMP expressed as total megabecquerels (or millicuries) and the concentration as megabecquerels per mL (or millicuries per mL) at the time of calibration; the expiration date and time; and the statement “Caution—Radioactive Material.” The labeling indicates that in making dosage calculations, correction is to be made for radioactive decay, and also indicates that the radioactive half-life of
153Sm is 46.3 hours. The labeling indicates that it should not be diluted or mixed with other solutions, that it is to be thawed at room temperature before administration, and that it is to be used within 8 hours of thawing.
Radionuclide identification  821
821 —
—
Samarium-153 decays by beta emission to stable Europium 153 as follows: 640 keV (30%), 710 keV (50%), and 810 keV (20%). The average emission energy is 233 keV. Its gamma-ray spectrum is identical to that of a specimen of known purity of
153Sm that exhibits major photopeaks having energies of 70 keV and 103 keV.
Bacterial endotoxins  85
85 —
—
The limit of endotoxin content is not more than 175/
V USP Endotoxin Unit per mL of Injection, in which
V is the maximum recommended total dose, in mL, at the expiration time.
Radionuclidic purity  821
821 —
—
Using a gamma ray spectrophotometer, determine the radionuclidic purity of the Injection: not less than 99.8% of the total radioactivity is present as Sm 153 at the time of expiry. The Europium 154 radioactivity is not more than 3.44 kBq per 37 MBq of Samarium 153 (or 0.0093% of total Samarium 153 at expiry). The sum of all other radionuclidic impurities is less than or equal to 0.1907% of the total Sm 153 at expiry.
Radiochemical purity  821
821 —
—
Mobile phase—
Transfer 8.0 g of sodium chloride, 0.2 g of monobasic potassium phosphate, 1.15 g of dibasic sodium phosphate, and 0.2 g of potassium chloride to a 1-liter volumetric flask, dilute with distilled water to volume, and mix. Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Chromatographic system
(see
Chromatography  621
621
)—A 10-mm × 40-mm glass chromatographic column is packed using gravity flow with a strong cation-exchange resin
* (prepared by mixing 5 g of resin with 25 mL of water in a suitable beaker) to a final resin volume of 0.5 mL.
Procedure—
Transfer the packed column to an ion-chamber dose counter to determine the background count for Sm 153. Apply about 10 µL of the Injection onto the column and place it in the ion-chamber counter, and record the total radioactivity. Elute the complexed radioactivity using about 20 mL of
Mobile phase, and record the radioactivity retained on the column. Subtract the background radioactivity from all measured radioactivity values. Calculate the percentage of complexed radioactivity in the portion of Injection taken by the formula:
100(
T 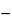 S /
S / 0.95) /
T,
in which
T is the total amount of radioactivity;
S is the quantity of free Sm-153 retained on the column; and 0.95 is the correction factor (5% of the uncomplexed Sm 153 passes through the column and into the Injection): not less than 99% of the Sm-153 is complexed by EDTMP.
Other requirements—
It meets the requirements under
Injections  1
1
, except that it is not subject to the recommendation on
Volume in Container.
Assay for radioactivity  821
821 —
—
Using a suitable counting assembly (see
Gamma-Emitting Radionuclides under the
Assay), determine the radioactivity in MBq (or mCi) per mL of Injection by use of a calibrated system: the activity is within ±10% of the labeled amount at the time of calibration.