Test solution—
Take a number of Capsules, equivalent to about 10 g of extract, open the Capsules using a suitable cutting instrument, and transfer the shells and contents to a suitable container. Transfer about 5 g, accurately weighed, to a 250-mL round-bottom flask, and evaporate in vacuum at a temperature not exceeding 50

. Add 50 mL of a solution prepared by dissolving 130 g of potassium hydroxide in 200 mL of water and diluting with methanol to 1000 mL. Attach a condenser, and reflux in a bath at 100

until a clear solution is obtained. Reflux for an additional 10 minutes, and cool by adding 50 mL of water through the condenser. Quantitatively transfer to a separation funnel, rinsing the flask with a total of 50 mL of water in small portions. Extract with 80 mL of ether, shaking for 30 seconds, and repeat twice.
[NOTE—If an emulsion forms, it can be eliminated by adding small quantities of methanol.
] Transfer the combined ether layers to a separation funnel, and wash with successive portions of 50 mL of water until a neutral washing is obtained.
[NOTE—If an emulsion forms, it can be eliminated by adding small quantities of methanol.
] Pass the ether extract through filter paper containing anhydrous sodium sulfate, wash the filter with 30 mL of ether, and evaporate to dryness in vacuum. Dissolve the residue in 2.0 mL of chloroform.
Apply separately 200 µL of this solution and 20 µL of a solution of
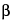
-cholestanol in chloroform (1 in 100) to a thin-layer chromatographic plate coated with 0.25-mm silica gel having an application zone that was previously dipped under 3 cm of a solution prepared by dissolving 13 g of potassium hydroxide in 20 mL of water and diluted to 1000 mL with methanol.
[NOTE—Allow the plate to dry, and heat it to 100

for 1 hour before use. The plate can be stored in a desiccator containing calcium chloride until the time of use.
] Develop with a solvent consisting of a mixture of hexanes and ether (7:3) until the solvent front has moved 17 to 19 cm. Keep the chamber temperature between 15

and 20

. Dry the plate in a current of warm air, then spray with an alkaline solution of 2,7-dichlorofluorescein in alcohol (0.2 in 100). Observe the plate under 366-nm wavelength light and identify the bands corresponding to the sterols by referring to the
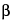
-cholestanol spot. Scrape off these bands and transfer them to a test tube. Add 10 mL of warm chloroform, and shake for 2 minutes with the aid of several glass beads. Filter the chloroform solution, wash the filter with chloroform, and evaporate the combined filtrate and washings to dryness in vacuum. Dissolve the residue with some drops of anhydrous acetone and evaporate in vacuum. Dry the residue in an oven at 105

for 15 minutes. Dissolve the residue in 0.2 mL of
Derivatizing reagent, and allow to stand for not less than 15 minutes at room temperature.
Procedure—
Proceed as directed for
Content of sterols under
Saw Palmetto Extract. The chromatogram of the
Test solution exhibits peaks for campesterol,
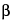
-sitosterol, and stigmasterol, identified by their retention times relative to the
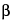
-sitosterol peak in the chromatogram of the
Standard solution.