Antimicrobial agent(s)—
It meets the requirements under
Antimicrobial Preservatives—Effectiveness  51
51
, and meets the labeled claim for content of the antimicrobial agent(s) as determined by the method set forth under
Antimicrobial Agents—Content  341
341
, except to use the following procedure when methylparaben and propylparaben are used as the antimicrobial agents.
Mobile phase—
Prepare a filtered and degassed mixture of methanol and water (70:30). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard preparation—
Dissolve accurately weighed quantities of
USP Methylparaben RS and
USP Propylparaben RS in methanol, and dilute quantitatively, and stepwise if necessary, with methanol to obtain a solution having known concentrations of about 1.2 and 0.12 mg per mL, respectively. Pipet 5 mL of this solution into a 50-mL volumetric flask, add by pipet 30 mL of methanol, dilute with water to volume, and mix.
Test preparation—
Pipet 1 mL of Injection into a 10-mL volumetric flask, add by pipet 7 mL of methanol, dilute with water to volume, and mix.
Chromatographic system
(see
Chromatography  621
621
)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm × 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the
Standard preparation as directed for
Procedure: the capacity factor,
k¢, is 0.52 for methylparaben and 1.05 for propylparaben, with a minimum separation factor (
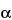
) of about 2.0.
Procedure—
Separately inject equal volumes (about 12 µL) of the
Standard preparation and the
Test preparation into the chromatograph by means of a suitable microsyringe or sampling valve, adjusting the specimen size and other operating parameters such that the peak obtained with the
Standard preparation is about 0.7 full scale. Record the chromatograms, and measure the height of the peaks, at identical retention times, obtained with the
Test preparation and the
Standard preparation, and calculate the concentration in mg per mL, in the portion of methylparaben or propylparaben taken by the formula:
C(HU / HS),
in which
C is the concentration, in mg per mL, of
USP Methylparaben RS or
USP Propylparaben RS in the
Standard preparation; and
HU and
HS are the peak heights obtained from the
Test preparation and the
Standard preparation, respectively.