Identification—
A:
Transfer 0.1 mL of Oral Solution to a small test tube, and add 0.1 mL of a freshly prepared mixture (1:1) of sodium alizarinsulfonate solution (1 in 1000) and zirconyl nitrate solution (1 in 1000) in 7 N hydrochloric acid: a yellow color is produced.
B:
If necessary, reduce the volume of a portion of it by heating on a steam bath to a reduced volume containing about 10 mg of sodium per mL: the solution so obtained responds to the tests for
Sodium  191
191
.
Assay—
[NOTE—Store all solutions, except
Buffer solution, in plastic containers.
]
Buffer solution—
Dissolve 57 mL of glacial acetic acid, 58 g of sodium chloride, and 4 g of (1,2-cyclohexylenedinitrilo)tetraacetic acid in 500 mL of water. Adjust with 5 N sodium hydroxide to a pH of 5.25 ± 0.25, dilute with water to 1000 mL, and mix.
Standard preparations—
Dissolve an accurately weighed quantity of
USP Sodium Fluoride RS quantitatively in water to obtain a solution containing 420 µg per mL. Each mL of this solution (
Standard preparation A) contains 190 µg of fluoride ion (10
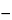 2 M
2 M). Transfer 25.0 mL of
Standard preparation A to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution (
Standard preparation B) contains 19 µg of fluoride ion per mL (10
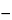 3 M
3 M). Transfer 25.0 mL of
Standard preparation B to a 250-mL volumetric flask, dilute with water to volume, and mix. This solution (
Standard preparation C) contains 1.9 µg of fluoride ion per mL (10
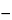 4 M
4 M).
Assay preparation—
Transfer an accurately measured volume of Oral Solution, equivalent to about 10 mg of fluoride, to a 500-mL volumetric flask, dilute with water to volume, and mix.
Procedure—
Pipet 20 mL of each
Standard preparation and of the
Assay preparation into separate plastic beakers each containing a plastic-coated stirring bar. Pipet 20 mL of
Buffer solution into each beaker. Concomitantly measure the potentials (see
pH  791
791
), in mV, of the solutions from the
Standard preparations and of the solution from the
Assay preparation, with a pH meter capable of a minimum reproducibility of ±0.2 mV and equipped with a fluoride-specific ion-indicating electrode and a calomel reference electrode.
[NOTE—When taking measurements, immerse the electrodes in the solution, stir on a magnetic stirrer having an insulated top until equilibrium is attained (1 to 2 minutes), and record the potential. Rinse and dry the electrodes between measurements, taking care to avoid damaging the crystal of the specific-ion electrode.
] Plot the logarithms of the fluoride-ion concentrations, in µg per mL, of the
Standard preparations versus potential, in mV. From the measured potential of the
Assay preparation and the standard response line, determine the concentration,
C, in µg per mL, of fluoride ion in the
Assay preparation. Calculate the quantity, in mg, of fluoride ion in each mL of the Oral Solution taken by the formula:
0.5(C / V),
in which
C is the determined concentration of fluoride, in µg per mL, in the
Assay preparation, and
V is the volume, in mL, of Oral Solution taken. Multiply the quantity of fluoride ion by 2.21 to obtain the quantity of NaF.