Limit of mercaptopurine—
Prepare solutions in dimethylformamide containing, respectively, 10 mg of Azathioprine Sodium for Injection per mL, 10 mg of
USP Azathioprine RS per mL, and 100 µg of
USP Mercaptopurine RS per mL. Apply 15 µL of the
USP Mercaptopurine RS solution and 5-µL portions of the other two solutions at points about 2 cm from the bottom edge of a thin-layer chromatographic plate (see
Chromatography  621
621
) coated with a 250-µm layer of microcrystalline cellulose. Allow the spots to dry, and develop the chromatogram in a suitable chamber, using butyl alcohol, previously saturated with 5 N ammonium hydroxide, as the solvent, until the solvent front has moved about 15 cm from the point of application. Remove the plate, air-dry, and locate the spots by viewing under short- and long-wavelength UV light: any spot in the chromatogram from azathioprine, other than the principal spot, is not more intense than the spot in the chromatogram obtained with
USP Mercaptopurine RS (3.0%).
Assay—
Standard preparation—
Transfer 25 mg of
USP Azathioprine RS, accurately weighed, to a 50-mL volumetric flask, dissolve in 2.5 mL of 0.1 N sodium hydroxide, dilute with water to volume, and mix. Pipet 10.0 mL of this solution into a 50-mL volumetric flask, dilute with 0.1 N sulfuric acid to volume, and mix.
Assay preparation—
Transfer the contents of 1 vial of Azathioprine Sodium for Injection with the aid of water to a 100-mL volumetric flask, dilute with water to volume, and mix. Pipet 10 mL of this solution into another 100-mL volumetric flask, dilute with 0.1 N sulfuric acid to volume, and mix.
Procedure—
Transfer 20 mL each of the
Standard preparation and the
Assay preparation, separately, to polarographic cells, and deaerate for 10 minutes with nitrogen that previously has been saturated with 0.1 N sulfuric acid. Blanket the solution with saturated nitrogen, insert the dropping mercury electrode of a suitable polarograph, and record the polarogram from
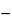
0.60 volt to
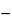
1.00 volt, using a saturated calomel electrode as the reference electrode. Determine the height of the diffusion current as the difference between the residual current and diffusion current plateau. Calculate the quantity, in mg, of azathioprine (C
9H
7N
7O
2S) in the volume of solution from the vial used for the
Assay preparation taken by the formula:
0.1C(id)U / (id)S,
in which
C is the concentration, in µg per mL, of
USP Azathioprine RS in the
Standard preparation; and (
id)
U and (
id)
S are the diffusion currents of the
Assay preparation and the
Standard preparation, respectively.